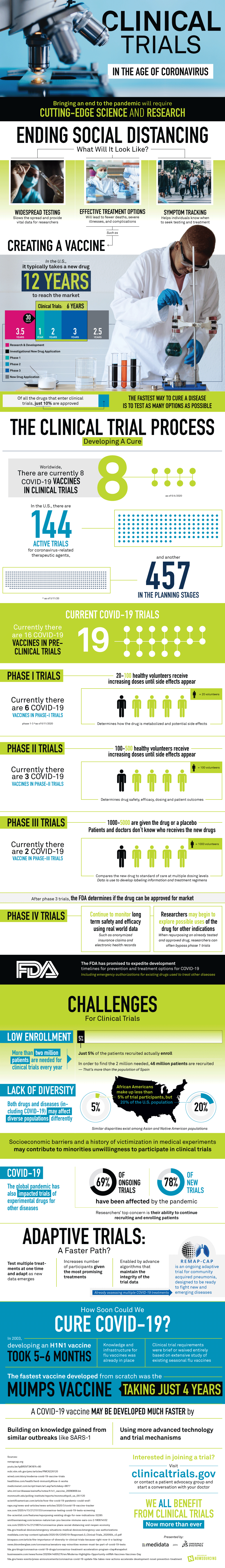
In order to develop a vaccine for COVID-19, a lot of time, money, and effort must be put in by scientists around the world, using cutting-edge research and science. Creating a new drug in the U.S. takes typically 12 years in development, 3 phases of clinical trials, and implementation. Around the world, 8 different COVID-19 vaccines are in clinical trials and in the U.S., there are 144 active trials for COVID-19-related therapeutic agents, and another 457 are still in the planning stages.
As of now, there are 16 COVID-19 vaccines in pre-clinical trials, each in varying stages of the trials. The 6 vaccines in Phase 1 trials are being tested on 20 to 100 healthy volunteers that will receive increasing doses of the vaccine until side effects start to appear. This phase is to determine how the drug is metabolized and the potential side effects it may bring. The 3 vaccines in Phase 2 trials are being tested on 100 to 500 healthy volunteers and the volunteers are again receiving increasing doses until side effects start to show. This test is to determine the drug’s safety, efficacy, dosing, and the outcomes on patients. The 2 COVID-19 vaccines in Phase 3 trials are given to 1000 to 5000 volunteers alongside a placebo. This test compares the new drug to standard of care multiple dosing levels – this data is then used to develop labeling information and treatment schedules. After these 3 trials, the FDA carefully determines if the drug can be approved to hit the market. Another fourth trial uses real world data long after the drug is released to truly determine how well the drug works and also how safe the drug actually is.
Learn more about the challenges that clinical trials are facing and how soon we might have the vaccine to COVID-19 here.